一. Asymmetric Catalysis (Asymmetric Hydrogenation, Asymmetric isomerizaiton of allylic alcohols)
1. Asymmetric Hydrogenation
Enantiomerically enriched secondary alcohols are versatile and valuable building blocks for the manufacture of biologically active compounds such as natural products and pharmaceuticals, and much efficient strategies have been developed by chemist for the synthesis of chiral alcohols. Among them, asymmetric hydrogenation ofprochiral ketones is considered as one of the most direct, efficient and beneficial methods to produce various optically active alcohols both from academic and industrial points of view, because this chemical method using dihydrogen and small amount of chiral transition metal complex is clean, operationally simple, economical and suitable for large-scale reactions.
Our group synthesized a new diphosphine ligand SunPhos in 2004 (Figure 1). After the geminal substituted groups (dimethyl, cyclopentyl or cyclohexyl) were introduced onto the SegPhos scaffold, the dihedral angles along the C2-axial (C1-C2-C3-C4) and C1-C2-C5-C6) were adjusted but the coordination environment remained.

Then we also exploited their applications in asymmetric hydrogenation of various ketones such as α-, β-keto acid derivatives, β-keto sulfones, β-ketophosphonates; and good results were obtained. We also have been focused on some more challenging substrates such as polyfunctionlized ketones including (E)-ethyl 2-oxo-4-arylbut-3-enoates, γ,δ-unsaturated β-keto esters; γ-heteroatom and/or δ-ketal substituted β-keto esters; 3-oxoglutaric acid derivatives, 3,5-diketo amides, and some tricky control of chemo- and enantioselectivities were achieved(Figure 2). More importantly, we were very interested in the potential applications of the hydrogenation methods for the synthesis of important pharmaceuticals and natural products.
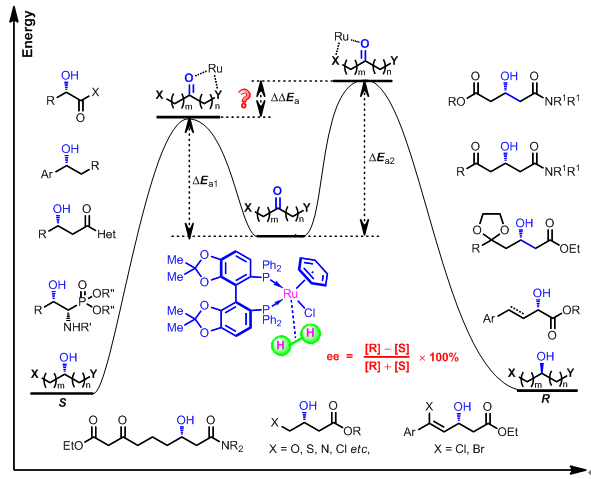
2. Asymmetric Isomerizaiton of Allylic Alcohols
Carbonyl compounds are key synthetic intermediates in the fields of medicine, pesticide, perfumes and cosmetics.In recent years, transition metal catalyzed Asymmetric isomerization of allylic alcohols to synthesize various carbonyl compounds has drawn much attention and been explored extensively, due to their high atomic economy and unique superiority.
二. Visible-light Catalyzed Reaction
Since 2013, Our group has applied a national key project about visible-light catalyzed reaction with the xiao’s group from Central China Normal University. We are devoted to develop a series of mild, efficient method in constructing organic molecules by visible-light catalyzed reaction. Now, we mainly focus on C-X bond activation, the construction of N heterocyclic compounds and so on. Some relevant works is under way.
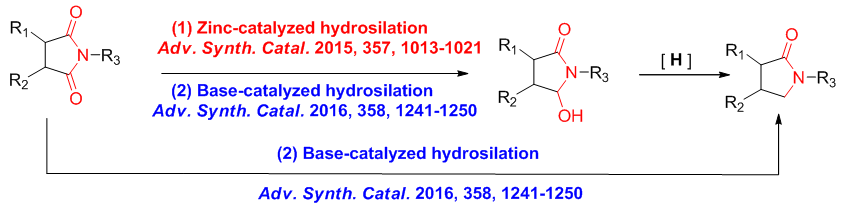
三. Hydrosilylation
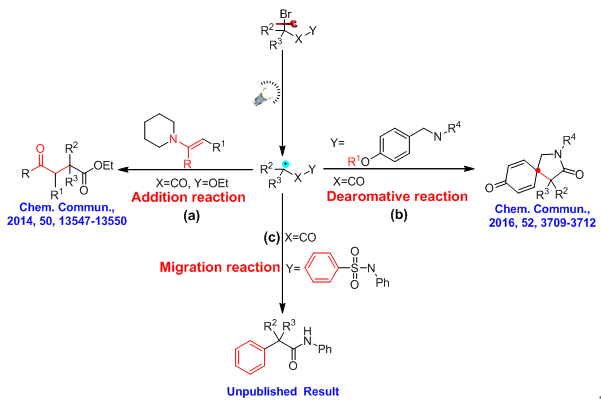
The reduction of carbonyl groups is an important transformation in organic synthesis, and the chemoselective reduction of multi-functionalized carbonyl compounds under mild conditions is challenging. The metal/Lewis acid-catalyzed hydrosilylation of carbonyl compounds have drawn much attention, due to the high chemoselectivity, environmentally benign and mild conditions. So using such mild and convient method to reduce imides and carboxylic acid derivatives arouses our interest.Our group have reported an efficient hydrosilylation of cyclic imides to ω-hydroxylactams using convenient zinc and base catalysts with excellent hemoselectivity and good functional group tolerance.
四. Other Transition Metal Catalysis
Our research group developed Several monophosphine ligands based on binaphthyl skeleton, which can regulate different substituents to regulate dihedral angle and electric charge of ligand. We successfully realized C-N and C-P and C-O cross-coupling reaction .